Multiple Choice
One resonance structure for OCN- ion is drawn below.What is the formal charge on each atom? 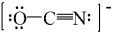
A) O atom = 0,C atom = 0,and N atom = 0
B) O atom = 0,C atom = 0,and N atom = -1
C) O atom = -1,C atom = 0,and N atom = 0
D) O atom = -1,C atom = -1,and N atom = +1
E) O atom = +1,C atom = 0,and N atom = -2
Correct Answer:

Verified
Correct Answer:
Verified
Q1: When both of the electrons in a
Q3: Use VSEPR theory to predict the electron-pair
Q9: In molecules,as bond order increases,<br>A) both bond
Q26: Use VSEPR theory to predict the molecular
Q52: The molecular geometry of a molecule whose
Q61: Use VSEPR theory to predict the molecular
Q90: What is the correct Lewis structure for
Q93: Which of the following has a Lewis
Q96: An atom of Se has _ valence
Q97: Which of the following is/are true concerning