Multiple Choice
Use the bond energies provided to complete the following statement.________ when all of the bonds in acetic acid (CH3COOH) are formed.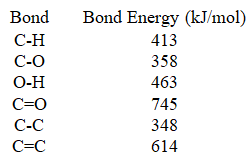
A) 3153 kJ/mol of energy is released
B) 3153 kJ/mol of energy is consumed
C) 2805 kJ/mol of energy is released
D) 2805 kJ/mol of energy is consumed
E) 2766 kJ/mol of energy is consumed
Correct Answer:

Verified
Correct Answer:
Verified
Q23: What is the formal charge on each
Q30: What are the approximate O-S-O bond
Q31: Which of the following elements is able
Q33: How many hydrogen atoms are needed to
Q35: Which of the following species will have
Q36: Which of the following has an incomplete
Q37: In which pair do <span
Q39: Which of the following is a correct
Q44: Which combination of atoms is most likely
Q72: Which combination of atoms is most likely