Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 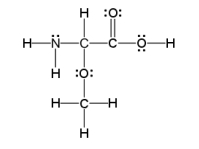
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Atomic orbitals combine most effectively to form
Q48: What is the molecular geometry around a
Q50: Benzene,C<sub>6</sub>H<sub>6</sub>,consists of a six member ring
Q51: Which of the following statements concerning valence
Q52: Which of the following statements concerning molecular
Q54: The hybridization of the nitrogen atom in
Q56: Which molecule will have the following valence
Q57: Diagram 9-1 The molecular orbital diagram
Q58: Diagram 9-1 The molecular orbital diagram
Q60: What is the maximum number of hybridized