Multiple Choice
What does the following figure represent? 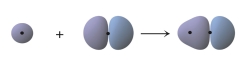
A) the overlap of two 1s orbitals to form a bond
B) the overlap of two 2p orbitals to form a bond
C) the overlap of two 2p orbitals to form a bond
D) the overlap of a 1s orbital and a 2p orbital to form a bond
E) the overlap of a 1s orbital and a 2p orbital to form a bond
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Draw a Lewis structure of xenon trioxide.What
Q32: Which of the following characteristics apply to
Q33: What is the hybridization of the sulfur
Q35: For which of the following molecules or
Q35: Refer to diagram 9-1.Identify the molecule with
Q36: Which diatomic molecule or ion has
Q38: For which of the following compounds is
Q38: The hybridization of the carbon atom in
Q40: In the NO<sub>2</sub><sup>-</sup> ion,each atom can
Q42: How many sigma ( <span