Multiple Choice
Which intermolecular force or bond is responsible for the density of H2O(s) being less than that of H2O( 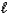 ) ?
) ?
A) London dispersion forces
B) hydrogen bonding
C) ionic bonding
D) covalent bonding
E) dipole/induced dipole forces
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: A molecule will always be polar if
Q24: The _ equation relates the equilibrium vapor
Q57: _ is a measure of the degree
Q68: When a water molecule forms a hydrogen
Q70: Which of the following statements concerning induced
Q72: A liquid has an enthalpy of vaporization
Q74: List all the intermolecular forces present in
Q77: If 3.21 g of water is sealed
Q78: At 75.0 <sup> <span class="ql-formula" data-value="\circ"><span
Q79: Which of the following statements concerning intermolecular