The Heat of Vaporization of Benzene,C6H6,is 30 CHow Much Energy in the Form of Heat Is Required
Multiple Choice
The heat of vaporization of benzene,C6H6,is 30.7 kJ/mol at its boiling point of 80.1 C.How much energy in the form of heat is required to vaporize 148 g benzene at its boiling point?
A) 0.207 kJ
B) 4.82 kJ
C) 16.2 kJ
D) 58.2 kJ
E) 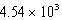 kJ
kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which one of the following sets of
Q5: On a relative basis,the weaker the intermolecular
Q12: A molecule will always be polar if
Q41: Which of the following compounds has a
Q60: Which one of the following molecules has
Q62: Sulfur dioxide has a vapor pressure
Q63: What intermolecular force or bond is primarily
Q63: When a glass tube with a small
Q65: For a particular liquid,raising its temperature from
Q67: Freon-113,C<sub>2</sub>Cl<sub>3</sub>F<sub>3</sub>,has an enthalpy of vaporization of