Multiple Choice
The Arrhenius equation, 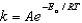 expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
A) 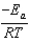
B) 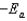
C) 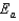
D) 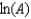
E) 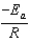
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: For the formation of 1 mol
Q23: Radioactive isotopes decay by _-order kinetics.
Q24: Consider the following proposed mechanism.If this mechanism
Q25: For the zero-order reaction below,a graph
Q28: Molecules must overcome a barrier called the
Q28: For the overall reaction<br>A + 2B
Q29: A first-order reaction is 40.0% complete
Q31: At a given temperature,a first-order reaction
Q32: How many mechanistic steps are depicted by
Q58: What is the name given to a