Multiple Choice
The equilibrium constant,Kc,for the decomposition of ammonium hydrogen sulfide is 1.8 10-4 at 25 C.NH4HS(s) 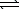 NH3(g) + H2S(g)
NH3(g) + H2S(g)
If excess NH4HS(s) is allowed to equilibrate at 25 C,what is the equilibrium concentration of NH3?
A) 3.2 10-8 M
B) 9.0 10-5 M
C) 1.8 10-4 M
D) 6.7 10-3 M
E) 1.3 10-2 M
Correct Answer:

Verified
Correct Answer:
Verified
Q10: If the reaction quotient,Q,is equal to K
Q23: The thermochemical equation for the formation
Q24: Sulfuryl chloride decomposes to sulfur dioxide and
Q25: At a given temperature,K = 0.021 for
Q26: For the reaction given below,2.00 moles of
Q30: Consider the reaction H<sub>2</sub> + I<sub>2</sub>
Q31: Write a balanced chemical equation which corresponds
Q32: Which of the following equilibria would not
Q49: Which of the following statements about the
Q58: If the reaction quotient,Q,is greater than K