Multiple Choice
Given the equilibrium constants for the following reactions:
4Cu(s) + O2(g)  2Cu2O(s) ,K1
2Cu2O(s) ,K1
4CuO(s)  2Cu2O(s) + O2(g) ,K2
2Cu2O(s) + O2(g) ,K2
What is K for the system
2Cu(s) + O2(g)  2CuO(s)
2CuO(s)
Equivalent to?
A) 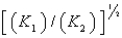
B) 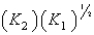
C) (K1) (K2)
D) 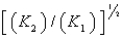
E) 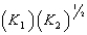
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Given the following equilibria,<br>PbBr<sub>2</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q18: What balanced equation is the following equilibrium
Q19: What is the K<sub>c</sub> equilibrium-constant expression for
Q20: An aqueous mixture of phenol and ammonia
Q21: Given the following equilibria,<br>Ni<sup>2+</sup>(aq)+ 2 OH<sup>-</sup>(aq)
Q23: The thermochemical equation for the formation
Q24: Sulfuryl chloride decomposes to sulfur dioxide and
Q25: At a given temperature,K = 0.021 for
Q26: For the reaction given below,2.00 moles of
Q49: Which of the following statements about the