Multiple Choice
What is the equilibrium constant for the following reaction,
HCO2H(aq) + CN-(aq) 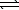 HCO2-(aq) + HCN(aq)
HCO2-(aq) + HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 10-4 and the acid dissociation constant for HCN is 4.0 10-10.
A) K = 1.00.The reaction favors neither the formation of reactants nor products.
B) K = 2.2 10-6.The reaction favors the formation of products.
C) K = 2.2 10-6.The reaction favors the formation of reactants.
D) K = 4.5 105.The reaction favors the formation of products.
E) K = 4.5 105.The reaction favors the formation of reactants.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which is the stronger Brønsted-Lowry acid,Fe(H<sub>2</sub>O)<sub>6</sub><sup>2+</sup> or
Q30: Consider the K<sub>a</sub> values for the
Q31: A solution has a hydroxide-ion concentration
Q32: Which is NOT an amphiprotic species in
Q32: Write a net ionic equation for the
Q33: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q34: What is the H<sub>3</sub>O<sup>+</sup> concentration in
Q38: Which of the following species
Q39: What is K<sub>a</sub> at 25°C for
Q69: Molecules or ions that can alternately behave