Multiple Choice
Which acid-base reaction results in an acidic solution?
A) HNO3(aq) + CsOH(aq) H2O( 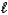 ) + CsNO3(aq)
) + CsNO3(aq)
B) HI(aq) + LiOH(aq) H2O( 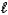 ) + LiI(aq)
) + LiI(aq)
C) HBr(aq) + NaOH(aq) H2O( 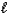 ) + NaBr(aq)
) + NaBr(aq)
D) H2SO3(aq) + LiOH(aq) 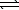 H2O(
H2O( 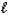 ) + LiHSO3(aq)
) + LiHSO3(aq)
E) HF(aq) + LiOH(aq) 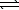 H2O(
H2O( 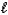 ) + LiF(aq)
) + LiF(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which is the stronger Brønsted-Lowry acid,Fe(H<sub>2</sub>O)<sub>6</sub><sup>2+</sup> or
Q22: Which of the following molecules or ions
Q42: The K<sub>a</sub> for the monoprotic acid
Q43: The pH of a solution at
Q44: What is the OH<sup>-</sup> concentration of
Q46: Rank the following in order of decreasing
Q47: Which of the following species is amphiprotic
Q48: Calculate the pH of a 0.04
Q49: According to the Brønsted-Lowry definition,an acid<br>A) increases
Q50: What is the hydroxide-ion concentration of