Multiple Choice
Potassium hydrogen phthalate (KHP) is used to standardize sodium hydroxide.If 35.39 mL of NaOH(aq) is required to titrate 0.8246 g KHP to the equivalence point,what is the concentration of the NaOH(aq) ? (The molar mass of KHP = 204.2 g/mol)
HC8H4O4-(aq) + OH-(aq) 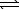 C8H4O42-(aq) + H2O(
C8H4O42-(aq) + H2O( 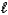 )
)
A) 0.02318 M
B) 0.05705 M
C) 0.0859 M
D) 0.1141 M
E) 0.1429 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: A 50.00-mL solution of 0.0797 M
Q45: What is the molar solubility of
Q47: If 25 mL of 0.750 M HCl
Q48: A 50.00-mL solution of 0.0350 M
Q50: If 500 mL of 1.2
Q51: The concentration of magnesium carbonate in a
Q52: What is the pH of a
Q53: You have 75.0 mL of 0.11
Q54: If 30 mL of 0.10 M
Q105: In which of the following solutions would