Multiple Choice
The solubility of barium carbonate (BaCO3) in water at 25°C is 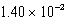 g/L.What is the Ksp of this sparingly soluble salt?
g/L.What is the Ksp of this sparingly soluble salt?
A) 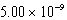
B) 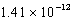
C) 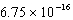
D) 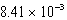
E) 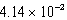
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: A 4.0 <span class="ql-formula"
Q11: What is the pH of a
Q12: A solution containing 10.mmol of CO<sub>3</sub><sup>2-</sup> and
Q13: What is the pH of a
Q14: What is the solution pH when
Q16: A 1.0-liter solution contains 0.25 M
Q17: What is the pH of a
Q18: When a weak base is titrated with
Q19: What is the value of the
Q20: When mixed in appropriate amounts,each of the