Multiple Choice
Consider the reaction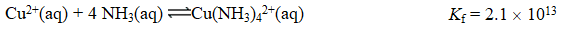
If the Ksp for Cu(OH) 2 is 2.2 10-20,what is the value of the equilibrium constant,K,for the reaction below?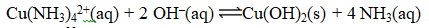
A) 1.0 10-33
B) 4.6 10-7
C) 2.1 1013
D) 2.2 106
E) 9.5 1032
Correct Answer:

Verified
Correct Answer:
Verified
Q51: A buffer is composed of 0.400 mol
Q58: If the ratio of acid to base
Q75: Given the two equilibria below,<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Given
Q77: A solution containing 10.mmol of CO<sub>3</sub><sup>2-</sup>and 5.0
Q79: Consider the titration of 300.0 mL
Q81: The solubility of copper(II)oxalate is 3.2
Q82: The hydroxide ion concentration of a saturated
Q83: The concentration of Pb<sup>2+</sup> in an
Q84: What color change is exhibited by phenolphthalein
Q86: A buffer contains 0.50 mol NH<sub>4</sub><sup>+</sup> and