Multiple Choice
Write a balanced chemical equation for the following reaction in an acidic solution.Cr2O72-(aq) + Ni(s) Cr3+(aq) + Ni2+(aq)
A) Cr2O72-(aq) + 3 Ni(s) + 14 H+(aq) 2 Cr3+(aq) + 3 Ni2+(aq) + 7 H2O( 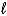 )
)
B) Cr2O72-(aq) + Ni(s) + 14 H+(aq) 2 Cr3+(aq) + Ni2+(aq) + 7 H2O( 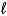 )
)
C) Cr2O72-(aq) + 3 Ni(s) 2 Cr3+(aq) + 3 Ni2+(aq) + O2-(aq)
D) Cr2O72-(aq) + Ni(s) + 14 H+(aq) 2 Cr3+(aq) + Ni2+(aq) + 7 H2O( 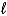 )
)
E) Cr2O72-(aq) + 3 Ni(s) + 7 H+(aq) 2 Cr3+(aq) + 3 Ni2+(aq) + 7 OH-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q66: The standard cell potential of the following
Q67: Write a balanced half-reaction for the
Q68: When the following oxidation-reduction reaction in
Q69: Use the standard reduction potentials below
Q70: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt=" Given:
Q72: What is the equilibrium constant (K)at 25°C
Q73: Assuming the following reaction proceeds in
Q74: Which of the following equations is a
Q75: Write a balanced half-reaction for the
Q76: The following electrochemical cell has a