Multiple Choice
If rG for the following reaction is -22.2 kJ/mol-rxn,calculate 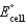 .
.
Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) Cu(s) + 2 AgCl(s)
A) -0.460 V
B) -0.115 V
C) +0.115 V
D) +0.230 V
E) +0.559 V
Correct Answer:

Verified
Correct Answer:
Verified
Q45: When a secondary battery provides electrical energy,it
Q74: Which of the following equations is a
Q75: Write a balanced half-reaction for the
Q76: The following electrochemical cell has a
Q77: Given the following standard reduction potentials,<br>Pb<sup>2+</sup>(aq)+
Q78: A current of 15.0 A is passed
Q80: Which of the following statements concerning a
Q81: Gold and platinum are commonly used as
Q82: Given:<br>2H<sup>+</sup>(aq)+ 2e<sup>-</sup> <span class="ql-formula" data-value="\to"><span
Q83: Calculate the standard reduction potential for