Multiple Choice
Which of the following compounds have +1 as a formal charge on the nitrogen atom? 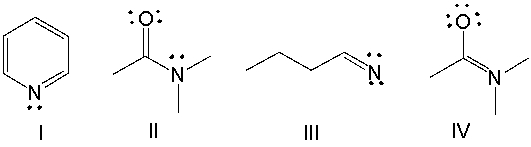
A) I
B) II
C) III
D) IV
E) Both I and II
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q130: Which of the following compounds contain an
Q131: Draw resonance structures for the following compound.
Q132: Which of the following pairs are resonance
Q133: Draw the Lewis structure for CH<sub>3</sub>C≡C(CH<sub>2</sub>)<sub>3</sub>C(CH<sub>3</sub>)<sub>3</sub>.
Q134: Which of the following is the correct
Q136: Draw significant resonance structures for the following
Q137: Determine the formal charges on each atom
Q138: What is the relationship between the following
Q139: What is a resonance hybrid?
Q140: Which of the following is the correct