Multiple Choice
The lone pair on nitrogen in the following compound is _______. 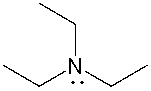
A) localized
B) delocalized
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: Which of the following is/are the most
Q99: How many hydrogen atoms are connected to
Q100: How many lone pairs of electrons are
Q101: Draw the curved arrow(s) for converting the
Q102: Which of the following is a correct
Q104: Draw all the constitutional isomers with a
Q105: Which of the following is/are the most
Q106: How many hydrogen atoms are connected to
Q107: Which of the following violates the rules
Q108: Draw the resonance hybrid for the following