Multiple Choice
The lone pairs on nitrogen in the following compound are _______. 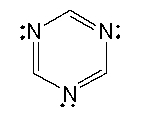
A) three localized
B) three delocalized
C) two localized and one delocalized
D) one localized and two delocalized
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Draw Lewis structure for the following compound.
Q2: Which of the following is/are the most
Q4: For the following equation, how many hydrogen
Q5: Norethynodrel, a component of the first combined
Q6: Draw all lone pairs of electrons for
Q7: What is the relationship between the following
Q8: Which of the following is the correct
Q9: What is the formal charge on the
Q10: The lone pairs on oxygen in the
Q11: Which of the following compounds contain an