Essay
For the following acid-base reaction, predict which side of the equilibrium is favored. Explain why. 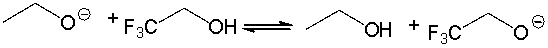
Correct Answer:

Verified
Favors the right side.
Both the base and...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Favors the right side.
Both the base and...
Both the base and...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q35: For the following reaction, identify the Lewis
Q36: Aspartic acid, an amino acid, has the
Q37: Which of the following compounds is most
Q38: Determine if H<sub>2</sub>O is a suitable reagent
Q39: For the following reaction, identify the Lewis
Q41: Tryptophan, an essential amino acid, is important
Q42: Which of the following compounds is most
Q43: Which of the indicated protons is most
Q44: In a Brønsted-Lowry acid-base reaction, the products
Q45: For the following reaction, identify the Lewis