Essay
Determine if NaNH2 is a suitable reagent to deprotonate the following compound. Explain why. 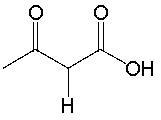
Correct Answer:

Verified
Yes.
The conjugate base is resonance sta...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Yes.
The conjugate base is resonance sta...
The conjugate base is resonance sta...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q67: For the following reaction label the acid,
Q68: In a Brønsted-Lowry acid-base reaction, the reactants
Q69: Which of the following compounds is most
Q70: What is a cation?<br>A) a negatively charged
Q71: Which of the following compounds is most
Q73: Identify a Brønsted-Lowry acid.<br>A) proton acceptor<br>B) proton
Q74: Which of the following compounds is more
Q75: Provide a curved arrow mechanism for the
Q76: For the following acid-base reaction, predict which
Q77: Draw the conjugate base of CH<sub>3</sub>C