Multiple Choice
Consider the following SN2 reaction, 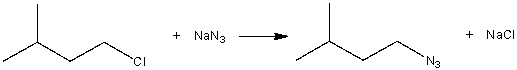 Assuming no other changes, what is the effect on the rate, if the concentration of NaN3 is tripled?
Assuming no other changes, what is the effect on the rate, if the concentration of NaN3 is tripled?
A) No effect
B) It would double the rate
C) It would triple the rate
D) It would increase four times
E) It would increase the rate six times
Correct Answer:

Verified
Correct Answer:
Verified
Q147: Which of the following is a secondary
Q148: Predict the product for the following S<sub>N</sub>1
Q149: Draw the E isomer of 2-methyl-3-heptene.
Q150: Predict the product for the following reaction.
Q151: Draw the mechanism and product for the
Q153: For the following reaction, label the nucleophile,
Q154: For the following dehydration, draw the structure
Q155: Predict the major product for the following
Q156: Which of the following is a substitution
Q157: Draw the isomer of 2-bromo-1,1,3-trimethylcyclohexane that would