Multiple Choice
In the molecule shown below, determine which of the highlighted C-H bonds (from a to e) is expected to have the lowest bond dissociation energy. 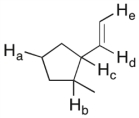
A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Propose a synthesis of 2-butyn-1-ol from propane.
Q61: Compound A has molecular formula C<sub>6</sub>H<sub>12</sub>. Upon
Q62: Propylene (propene) undergoes free radical polymerization with
Q63: Thermal cracking of butane can produce ethyl
Q64: Propose a synthesis of 2-methylpropene from 2-methylpropane.
Q66: Propose a synthesis of polyethylene from ethane.
Q67: Predict the product(s) of the following reaction.
Q68: How many constitutional isomers are possible if
Q69: Which of the following is an example
Q70: A bromine radical can add to the