Essay
Predict the product for the following reaction and explain how you can use IR spectroscopy to monitor the progress of the reaction. 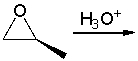
Correct Answer:

Verified
 _TB4454_00 A new abs...
_TB4454_00 A new abs...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q83: Mass spectrometry is primarily used to determine:<br>A)
Q84: Following are the IR spectrum and mass
Q85: Provide a molecular formula that is consistent
Q86: Which of the following compounds will have
Q87: Calculate the degree of unsaturation for C<sub>9</sub>H<sub>11</sub>N.<br>A)
Q89: In mass spectrometry using the electron impact
Q90: Calculate the degree of unsaturation for C<sub>14</sub>H<sub>14</sub>N<sub>2</sub>O.<br>A)
Q91: Rank absorption of the indicated bonds in
Q92: Which of the m/z values correspond to
Q93: Provide a molecular formula that is consistent