Solved
Which One of the Following Represents the Lowest Energy -Bonding Molecular Orbital of 1,3-Butadiene?
A) I
B) II
Multiple Choice
Which one of the following represents the lowest energy -bonding molecular orbital of 1,3-butadiene? 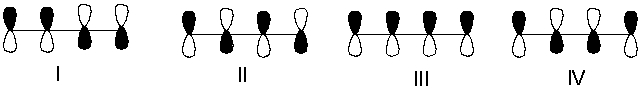
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q128: How many electrons does the HOMO of
Q129: Which one of the following dienes will
Q130: The addition of a _ bond improves
Q131: Predict the product for the following Diels-Alder
Q132: Absorption of UV-visible radiation by a molecule
Q134: Provide the reagents necessary to carry out
Q135: Predict the major product for the following
Q136: Which one of the following dienes is
Q137: Dienes with alternate π and σ bonds
Q138: Which of the following compounds has