Solved
Which One of the Following Represents the Highest Energy -Antibonding Molecular Orbital of 1,3-Butadiene?
A) I
B) II
Multiple Choice
Which one of the following represents the highest energy -antibonding molecular orbital of 1,3-butadiene? 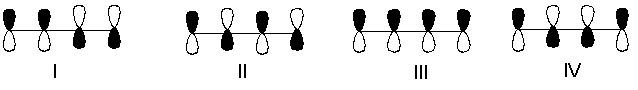
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: Classify the following compounds as having cumulated,
Q57: Provide the structure for (E)-1,3-pentadiene.
Q58: Identify the conjugated diene(s).<br>A. 4-methyl-1,3-heptadiene<br>B. 3-methyl-1,5-heptadiene<br>C. 2-methyl-2,4-heptadiene<br>D.
Q59: What is the IUPAC name for the
Q60: Which of the following compounds is an
Q62: How many electrons does the HOMO of
Q63: Which one of the following dienes will
Q64: Provide the structures of A, B and
Q65: Predict the product for the following Claisen
Q66: The following product is formed by an