Multiple Choice
Evaluate the Lewis structure below and determine its legitimacy. 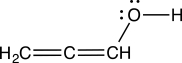
A) The structure is legitimate.
B) The structure is not legitimate, because the oxygen does not have an octet.
C) The structure is not legitimate, because the formal charges are not shown.
D) The structure is not legitimate, because the middle carbon lacks an octet.
E) The structure is not legitimate, because the leftmost carbon is missing a lone pair.
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Which condensed formula contains an aldehyde functional
Q29: An atom of which element would have
Q30: Amino acids are important building blocks in
Q31: Are the Lewis structures for sulfuric acid
Q32: The evolution of a chemical bond can
Q34: Which condensed formula contains an ester?<br>A)(CH<sub>3</sub>CH<sub>2</sub>)2O<br>B)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH<br>D)CH<sub>3</sub>CH<sub>2</sub>OH<br>E)CH<sub>3</sub>COH
Q35: Identify the functional groups present in the
Q36: Compare Structure A with Structures B,C,and D.Is
Q37: Identify which carbon atom in the molecule
Q38: (a)Sulfuric acid,H<sub>2</sub>SO<sub>4</sub>,is an important strong oxo acid