Essay
The six unique hydrogen atoms in the molecule below are labeled a-f.Suppose we individually replace each of these unique hydrogen atoms with a hydroxyl group (-OH)and draw a new molecule of formula C6H10O2.(In the cases of Ha,Hb,and Hc,substitute only one designated H with an OH.)Which of the new -OH groups would have localized lone pairs on oxygen? Which of the -OH groups would have a delocalized lone pair? Finally,for each molecule,identify the new functional group created. 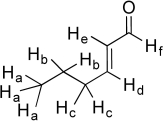
Correct Answer:

Verified
 * Ha replacement by OH yields an alcohol...
* Ha replacement by OH yields an alcohol...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q18: Which electron configuration is correct for a
Q19: A compound with a molecular formula of
Q20: For which of the following <font face="symbol"></font>amino
Q21: How many total valence electrons are used
Q22: Using a chemical reaction to convert an
Q24: When two Lewis structures are related as
Q25: Consider a peptide bond formed from the
Q26: Which point on the following diagram can
Q27: Which of the following Lewis structures violates
Q28: Which condensed formula contains an aldehyde functional