Multiple Choice
Styrene is an important industrial chemical in the generation of various plastics.Determine how many bonding molecular orbitals are created during the LCAO process to accommodate the π electrons in styrene. 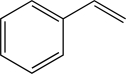
A) Two
B) Four
C) Six
D) Eight
E) Ten
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Draw line structures of a molecule with
Q4: One of the following statements about the
Q5: For the given molecule,an amino alcohol,how many
Q6: Rank the carbon atoms in order of
Q7: Tropyllium bromide is an ionic organic compound
Q9: Below are two reasonable acyclic structures for
Q10: Which two atomic orbitals overlap to form
Q11: Which two atomic orbitals overlap to form
Q12: Boat A and Boat B start on
Q13: Given below are 2s,2p,sp,sp<sup>2</sup>,and sp<sup>3</sup> orbitals,all drawn