Multiple Choice
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals represents the hybrid orbital with the lowest effective electronegativity? 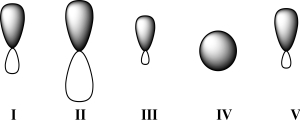
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Boat A and Boat B start on
Q13: Given below are 2s,2p,sp,sp<sup>2</sup>,and sp<sup>3</sup> orbitals,all drawn
Q14: The molecule benzene,which features a conjugated ring
Q15: Which functional group has two sp<sup>2</sup>-hybridized oxygen
Q16: Formaldehyde,CH<sub>2</sub>O,is a biological preservative and the simplest
Q18: Boat A follows Boat B across a
Q19: Select which of the following molecules have
Q20: An atom's electrons have a high probability
Q21: Why is a σ bond formed from
Q22: Sketch an orbital picture of ethylene,H<sub>2</sub>C <img