Multiple Choice
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals represents the hybrid orbital with the greatest effective electronegativity? 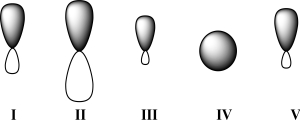
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Q30: How many total electrons reside in molecular
Q31: Tubulysin D is a peptide-based marine natural
Q32: Which of the following functional groups has
Q33: Which characteristics describe the bonding MO for
Q34: Amide bonds join amino acids together to
Q36: Given below are 2s,2p,sp,sp<sup>2</sup>,and sp<sup>3</sup> orbitals,in random
Q37: Which C<font face="symbol"></font><font face="symbol"></font><font face="symbol"></font>C bond in
Q38: What abbreviation is used to designate the
Q39: Which two carbon atoms participate in the
Q40: Draw line structures of a molecule with