Essay
Consider the hybrid structure below and the labeling scheme provided.Draw the most stable resonance contributor.Identify the hybridization and VSEPR geometry of each atom. 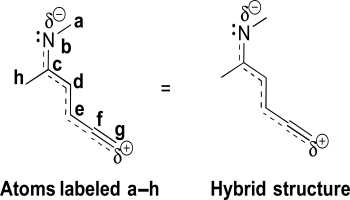
Correct Answer:

Verified
The neutral structur...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q19: Select which of the following molecules have
Q20: An atom's electrons have a high probability
Q21: Why is a σ bond formed from
Q22: Sketch an orbital picture of ethylene,H<sub>2</sub>C <img
Q23: Which C<font face="symbol"></font><font face="symbol"></font><font face="symbol"></font>C bond in
Q25: For one or more of the following
Q26: Given below are 2s,2p,sp,sp<sup>2</sup>,and sp<sup>3</sup> orbitals,all drawn
Q27: Compare the p orbital orientation and overlap
Q28: Which two atomic orbitals overlap to form
Q29: Which two carbon atoms participate in the