Short Answer
Explain why Compound A is more acidic than Compound B.Use resonance structures to explain your answer. 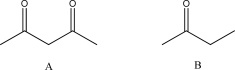
Correct Answer:

Verified
After Compound A is deprotonated,the neg...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
After Compound A is deprotonated,the neg...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q6: What is the correct proton transfer mechanism
Q7: Rank the following carbocations in order of
Q8: Supply the conjugate acid for the following
Q9: Which of the following protons has the
Q10: Which of the following resonance structures contributes
Q12: Rank the acidity of the following compounds
Q13: Rank the following compounds in order of
Q14: Supply the conjugate base for the following
Q15: Draw all the resonance structures for the
Q16: Which of the following is least basic?