Short Answer
For the following SN2 reaction,what would happen to the rate if the concentrations of both A and B were tripled? 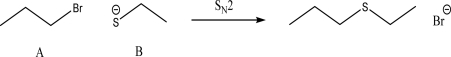
Correct Answer:

Verified
The reaction rate wo...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
The reaction rate wo...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q3: Draw the product(s)of the following E1 reaction.
Q4: Draw the product(s)of the following E1 reaction.
Q5: With regard to rate laws,provide the order
Q6: Which of the following steps could be
Q7: Give the rate law and order for
Q9: Draw the product(s)of the following S<sub>N</sub>1 reaction.
Q10: The following compound can undergo two successive
Q11: What is the product of the following
Q12: Which is true of a free energy
Q13: The following mechanism has an unreasonable step.Indicate