Short Answer
Name two bases that are strong enough to deprotonate the following alkyne. 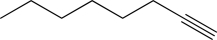
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Show the starting material needed to complete
Q2: Give the product and mechanism for the
Q4: Rank the following leaving groups' ability to
Q5: What is the major product of the
Q6: Rank the following nucleophiles in order of
Q7: Show the most likely product of the
Q8: Which attacking species would favor an E2
Q9: Which mechanism(s)would be favored under the following
Q10: Which mechanism(s)can occur when the following alkyl
Q11: Circle the solvent(s)that would speed up an