Multiple Choice
What is the rate-determining step in the reaction shown below? 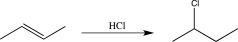
A) Nucleophilic addition
B) Coordination
C) Proton transfer
D) Electrophilic addition
E) Bimolecular nucleophilic substitution
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Draw the major product that would be
Q2: What would be the major product of
Q3: What is the most stable carbocation intermediate
Q4: Show how the following compound could be
Q6: When the following molecule is treated with
Q7: Identify the electron-poor species that participates in
Q8: Briefly explain why the hypothetical reaction below
Q9: Give an example of a compound containing
Q10: The electrophilic addition step in the reaction
Q11: Draw the most stable carbocation intermediate formed