Multiple Choice
What is true about the free energy diagram for the following reaction? 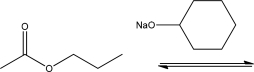
A) The first step of this reaction is the rate-determining step.
B) The free energy diagram for this reaction has three different transition states.
C) The overall products for this reaction include a carboxylic acid.
D) The first step of this reaction is exothermic.
E) The second step of this reaction is irreversible.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Supply two possible starting materials for the
Q16: What is the most likely product of
Q17: What is the most likely product of
Q18: What reagent(s)will complete the following reaction? <img
Q19: Explain why the following reaction energetically favors
Q21: Give three possible starting materials for the
Q22: Which of the following compounds will not
Q23: What reagent(s)will best complete the following reaction?
Q24: Give the mechanism and product for the
Q25: Give the most likely product of the