Multiple Choice
Which of the following compounds would undergo hydrolysis at the highest rate?
A) 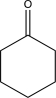
B) 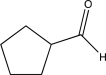
C) 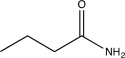
D) 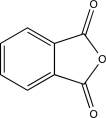
E) 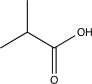
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Which of the following compounds would produce
Q18: Indicate which of the following molecules will
Q19: What is the major product of the
Q20: The acid-catalyzed transesterification reaction shown below is
Q21: What is the major product of the
Q23: The reaction below is an example of
Q24: Propose a synthetic route to carry out
Q25: Rank the following in order of increasing
Q26: Predict the major product of the following
Q27: Show how you would carry out the