Multiple Choice
Which of the following correctly explains how sulfuric acid catalyzes the reaction shown below? 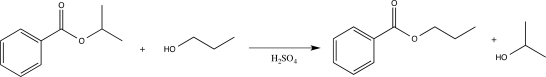
A) The acid increases the rate of nucleophile elimination.
B) The acid increases the nucleophilicity of the alcohol.
C) The acid donates a proton to the alcohol.
D) The acid converts the ester into a carboxylic acid.
E) The acid activates the carbonyl carbon and makes it more electrophilic.
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Which of the following elementary steps is
Q37: Fill in the missing starting material for
Q38: Determine the product of the following sequence
Q39: Show how the following could be synthesized
Q40: Identify the N-terminus,the C-terminus,and the peptide bonds
Q42: What is the product of the following
Q43: Determine the product(s)of the following reaction. <img
Q44: Draw a complete,detailed mechanism for the reaction
Q45: Which of the following is an intermediate
Q46: Fill in the boxes with an appropriate