Multiple Choice
In the following titration curve,what does the inflection point represent? 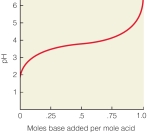
A) pH of solution equals pKa of weak acid
B) concentration of weak acid and conjugate base are equal
C) the pH where the solution would function most effectively as a buffer
D) the weak acid is 50% protonated,50% deprotonated
E) all of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following is the conjugate
Q3: Glutamic acid contains two carboxylic acid groups
Q9: If a buffer is made with the
Q11: If gastric juice has a pH of
Q12: Lysine contains two amine groups (pK<sub>a</sub> values
Q17: Imidazole is a commonly used buffer in
Q18: Which of the following would likely form
Q19: Formic acid is the active agent in
Q21: Given the pK<sub>a</sub> values for phosphoric acid
Q25: Which of the following is the most