Short Answer
What kind of molecular orbital (σ,σ*,π,or π*)results when the two atomic orbitals shown below interact in the manner indicated? 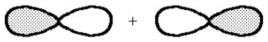
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: Give the number of nonbonding lone pairs
Q17: BF<sub>3</sub> has a dipole moment of zero.Propose
Q18: The Kekulé structure of pentane is shown
Q19: What is the CNN bond angle in
Q20: How many unpaired electrons are present in
Q22: What orbitals are used to form the
Q23: What type of bonding is most important
Q24: Give the hybridization,shape,and bond angle for the
Q25: Which of the following molecules has a
Q26: Give the hybridization,shape,and bond angle for each