Short Answer
What kind of molecular orbital (σ,σ*,π,or π*)results when the two atomic orbitals shown below interact in the manner indicated? 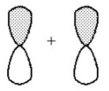
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Which of the following covalent bonds has
Q42: How many distinct and degenerate p orbitals
Q44: Identify the compound with the weakest bond.<br>A)H<sub>2</sub><br>B)HF<br>C)HCl<br>D)HBr<br>E)HI
Q45: Draw the Lewis structure for CH<sub>3</sub>N<sub>2</sub><sup>+</sup>.
Q47: The hydrogen-halogen bond becomes _ and _
Q49: A molecule of acetonitrile CH<sub>3</sub>CN contains _
Q50: The N-H single bond in methyl amine
Q51: What kind of molecular orbital (σ,σ*,π,or π*)results
Q61: Atoms with the same number of protons
Q131: Consider the interaction of two hydrogen 1s