Short Answer
What kind of molecular orbital (σ,σ*,π,or π*)results when the two atomic orbitals shown below interact in the manner indicated? 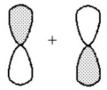
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: Draw the Kekulé structure for each of
Q65: The lone-pair electrons of the methyl anion
Q66: Identify the compound(s)that have a nonzero dipole
Q67: In 2015,the European Space Agency's Philae Lander
Q68: Both sigma (σ)and pi (π)bonds can be
Q70: Which of the following statements correctly describes
Q71: Nitrous oxide N<sub>2</sub>O,also known as laughing gas
Q72: What kind of molecular orbital (σ,σ*,π,or π*)results
Q73: Provide the mathematical equation for the dipole
Q74: Several volatile compounds are responsible for the