Essay
What is the product of the following Lewis acid-base reaction? 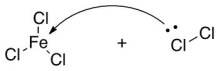
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which of the following ions is the
Q18: HCN has a pKa = 9.1.What form
Q19: Identify the compound with the highest pK<sub>a</sub>.<br>A)CH<sub>3</sub>CH<sub>3</sub><br>B)HCCH<br>C)CH<sub>2</sub>CH<sub>2</sub><br>D)CH<sub>3</sub>OH<br>E)CH<sub>3</sub>NH<sub>2</sub>
Q20: Which of the following species cannot function
Q23: At what pH will 25% of a
Q24: What would be the conjugate acid in
Q25: When a small amount of hexanoic acid
Q26: The conjugate acid of H<sub>2</sub>O is<br>A)H<sub>3</sub>O<sup>-</sup>.<br>B)H<sub>3</sub>O.<br>C)H<sub>3</sub>O<sup>+</sup>.<br>D)HO<sup>-</sup>.<br>E)H<sub>2</sub>O<sup>+</sup>.
Q27: Draw a resonance contributor and the resonance
Q94: Consider the set of compounds, NH<sub>3</sub>, HF,