Multiple Choice
Which of the following would have the highest boiling point?
A) CH3CH2-O-CH2CH2-O-CH3
B) CH3-O-CH2CH2CH2-O-CH3
C) HO-CH2CH2CH2CH2-OH
D) CH3CH2-O-CH2-O-CH2CH3
E) 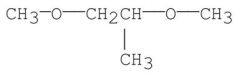
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: In the chair conformation of cyclohexane,how many
Q33: What is polarizability and how is it
Q34: Which of the compounds below will form
Q36: Which of the following compounds does not
Q38: Identify the number of tertiary carbons in
Q39: Give the structure of isopentyl alcohol.
Q40: Draw the Newman structure for the most
Q41: Which of the following has the lowest
Q42: Draw the most stable conformation of trans-1-tert-butyl-3-methylcyclohexane.
Q67: Which intermolecular force is primarily responsible for