Short Answer
Consider the reaction coordinate diagram shown.Which step has the greatest rate constant in the forward direction? 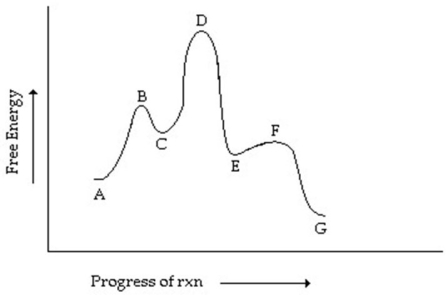
Correct Answer:

Verified
Step 3 (E ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Step 3 (E ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q23: Consider the reaction coordinate diagram shown.What is
Q24: Consider the one-step conversion of F to
Q25: Consider the reaction coordinate diagram shown.Which step
Q26: Consider the conversion of C to D
Q27: The Arrhenius equation models how the rate
Q29: What is the activation energy for the
Q30: An increase in which of the following
Q31: Based on the following energy diagram,which compound,A
Q32: Which of the following correctly describes the
Q33: Draw the structure of propyl vinyl ether.