Multiple Choice
Which of the following pairs are resonance structures? 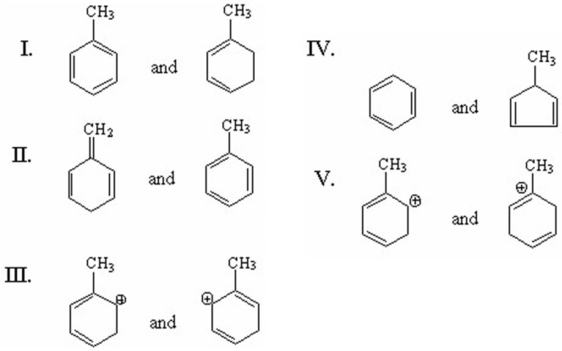
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: Give the hybridization,shape,and bond angle of a
Q75: What is the product of the following
Q76: Which of the following is the strongest
Q77: Draw the s-trans conformation of (2E,4Z)-4-methyl-2,4,-heptadiene.
Q78: Draw the s-cis conformation of (2E,4Z)-4-methyl-2,4,-heptadiene.
Q80: Draw the major product of the reaction.
Q82: Draw the important resonance contributing forms for
Q84: Which of the following is the weakest
Q105: When cycloheptatriene is deprotonated, an anion with
Q110: Classify cyclopentadienyl cation as aromatic, antiaromatic, or