Short Answer
Does the following structures represent different compounds or resonance contributors? 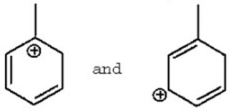
Correct Answer:

Verified
They are r...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
They are r...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q121: Aromatic molecules contain _ π electrons.<br>A)no<br>B)4n +
Q121: Consider the hydrogenation reaction of each compound
Q122: Show the missing reagents A and the
Q123: What diene and dienophile should be used
Q124: Which of the following is the strongest
Q125: Provide the structure of the major organic
Q127: Which of the following is the strongest
Q128: Due to electron delocalization,one would predict that
Q130: Provide the major organic product. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1831/.jpg"
Q131: The delocalized π system of benzene is