Multiple Choice
Why is the alkyl halide below not capable of undergoing an E2 reaction upon treatment with sodium ethoxide? 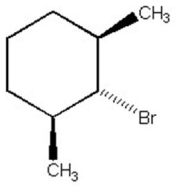
A) Br- is too poor a leaving group.
B) The substrate is too hindered.
C) Too much angle strain would be present in the alkene product.
D) Sodium ethoxide is a poor base to use in E2 reactions.
E) The C-H and C-Br bonds which need to break cannot achieve an anti-periplanar orientation.
Correct Answer:

Verified
Correct Answer:
Verified
Q117: Provide the structure of the major organic
Q118: Which of the following solvents is protic?<br>A)
Q119: Which of the following correctly describes SAM,a
Q120: In the S<sub>N</sub>1 hydrolysis mechanism of (CH<sub>3</sub>)<sub>3</sub>CBr,there
Q121: Predict the mechanism of the reaction and
Q123: Which of the following halides is most
Q124: Provide the structure of the major organic
Q125: The hydrolysis of tert-butyl chloride proceeds more
Q126: Provide the major organic product(s)in the reaction
Q127: Which of the following correctly reflects relative