Essay
Consider the following monobromination reaction,then answer the following questions. 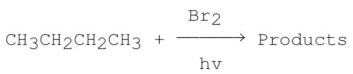 a)Give the structures and the IUPAC names for the products.
a)Give the structures and the IUPAC names for the products.
b)Give the common names for the products.
c)Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:  d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e)Propose a step-by-step mechanism for the major product only.
f)Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g)Does the transition state of the RDS resemble more closely the reactants or the products?
h)Would the value of the activation energy be different for different alkanes? Explain.
i)Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
Correct Answer:

Verified
a)  b)
b)  c)ΔH° for 2-bromobutane formatio...
c)ΔH° for 2-bromobutane formatio...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q49: Which of the following reactions is a
Q51: Which of the following products result from
Q52: Which of the following represents the best
Q53: The major type of reactions that alkanes
Q55: Provide the major organic product of the
Q56: What sequence of reagents can be used
Q57: An unknown sample is suspected of being
Q58: Give the best product for the following
Q59: Predict the major monobromination product in the
Q72: What C<sub>5</sub>H<sub>12</sub> isomer will give only a